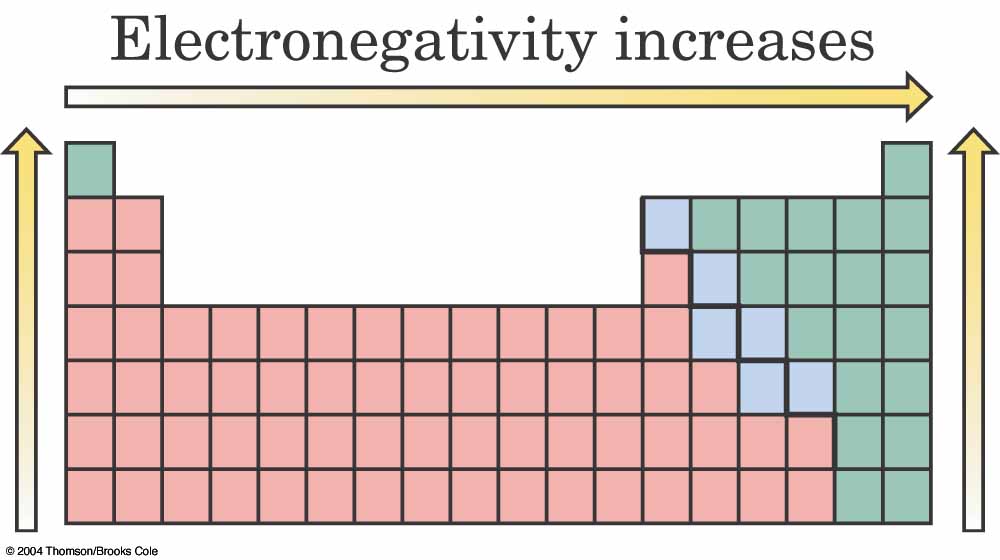
In actuality, most bonds lie somewhere between the
extremes of ionic and covalent bonds.
What governs the kind of bond
is an atom's electronegativity --
how strongly it wants to keep/add electrons.

Can you figure out why electronegativity
increases in the indicated directions?