http://www.eso.org/seaspace/water/water2.html
So why not HF and NH3? Those two compounds have
many (but not all) of the same properties that
water does.
But, among other things, they are not stable at
Earth's temperatures.
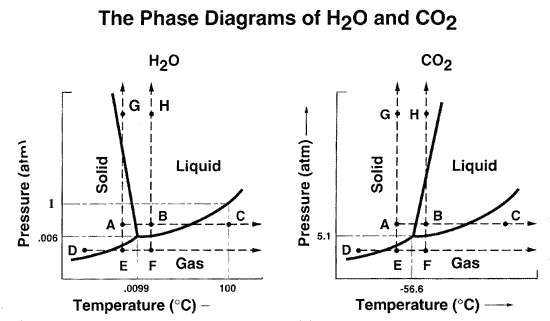
Water has an interesting phase diagram:

http://www.eso.org/seaspace/water/water2.html
This says that the liquid state of water is,
surprisingly, more compact than the solid
state (because if you push on water hard enough,
you transition from solid to liquid at a constant
temperature). This is another way of saying
the solid water is less dense than liquid
water: ice floats.
Also: at sufficiently low pressures, no
liquid water can exist. Where does Mars
fall on this plot?
However, it might be a case in which the best fit
on the early Earth was water, but that under other
conditions, other compounds are more suitable than
water. For example: Titan's methane cycle,
which we will talk about next week.