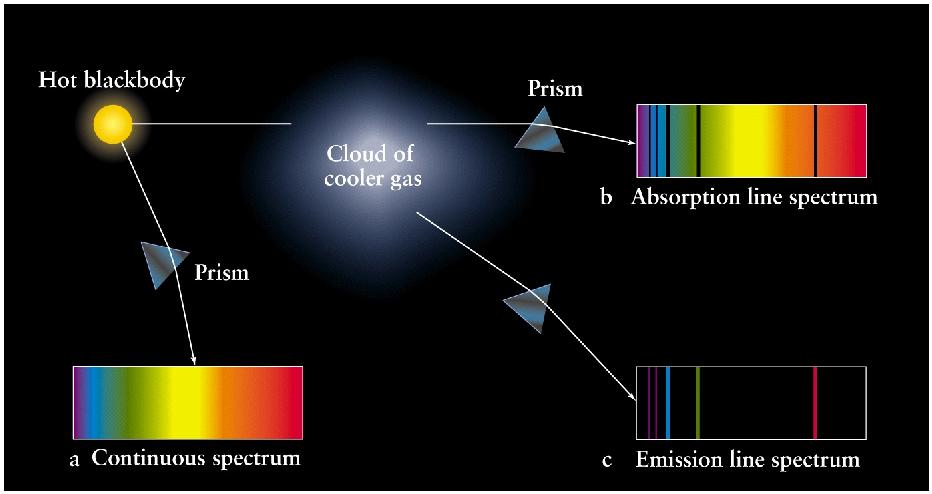
After some amount of time, electrons
in excited energy levels drop back
down to their original levels.
The energy released by electrons dropping
down is the same as the energy required
to excite them to their higher energy
levels, so the atom emits radiation at
exactly the same wavelength as it absorbed
earlier.
