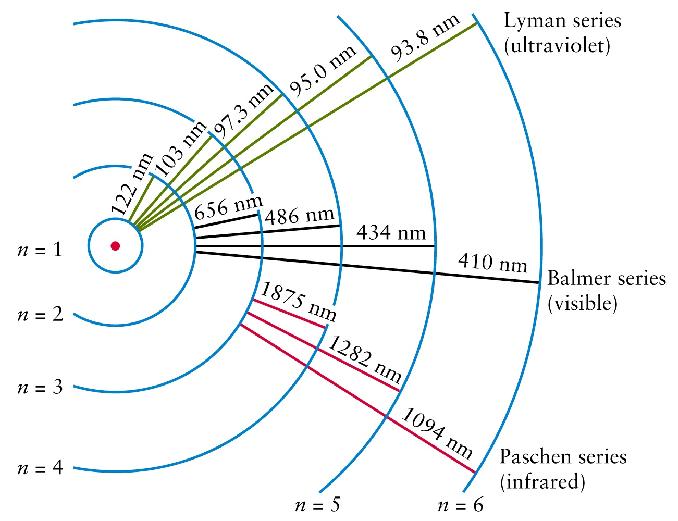
Atoms are constantly being bombarded
by energy.
Sometimes, the amount of energy that strikes
an atom is exactly equal to the amount of
energy it takes to move an electron
to a higher energy level.
That energy is absorbed, and the electron
moves up.
